COVID-19 illustration. Photo: Deposit Photos
The question of “do I or don’t I have COVID-19” may now be easy to answer. More options announced for at-home screening of the coronavirus are now available.
The U.S. Food and Drug Administration (FDA) authorized several over-the-counter (OTC) tests without a prescription when used for serial (frequent) screening.
In addition to the tests authorized for OTC use, one serial screening test was approved for use in a point-of-care (POC) setting without a prescription. An additional screening test was authorized for POC use with a prescription.
What Does All of This Mean?
The addition of the OTC and POC tests for screening will give schools, workplaces, communities, and others several options for serial screening tests that are accurate and reliable. These authorizations follow the FDA’s recent actions to advance OTC and other screening test development.
“Screening testing, especially with the over-the-counter tests, is an important part of the country’s pandemic response. Many schools, workplaces, communities, and other entities are setting up testing programs to quickly screen for COVID-19.
“With the FDA’s authorization of multiple tests, the public can be assured these tests have met our scientific standards for emergency use authorization. As we’ve said all along if it’s a good test, we’ll authorize it,” said Jeff Shuren, M.D., J.D., director of the FDA’s Center for Devices and Radiological Health.
“The FDA has taken many steps to support test development throughout the pandemic, including authorizing tests quickly, offering many avenues for test developers to work with us to get their tests on the market, if shown to be accurate and reliable, and issuing enforcement policies for COVID-19 tests. As the pandemic has progressed, we have worked with test developers wishing to add screening claims.”
In total, the FDA has authorized three tests with serial screening claims (testing asymptomatic individuals multiple times on a routine basis).
Specific tests authorized are:
 1. Quidel QuickVue At-Home OTC COVID-19 test – authorized for OTC at-home serial screening
1. Quidel QuickVue At-Home OTC COVID-19 test – authorized for OTC at-home serial screening
Product description from Quidel:
New QuickVue® At-Home OTC COVID-19 Test for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two (or three days) with at least 24 hours (and no more than 36 hours) between tests.
This test is authorized for nonprescription home use with self-collected (unobserved) direct anterior nares (NS) specimens from individuals aged 14 years and older or with adult-collected anterior NS samples from individuals aged 2 years or older.
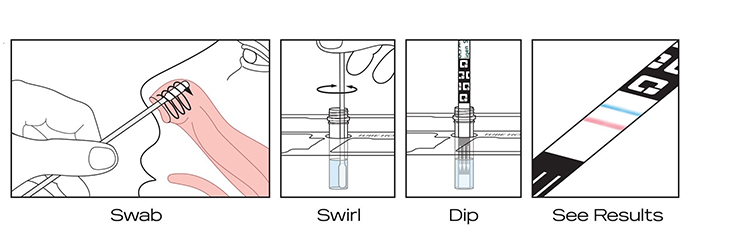
Illustration: Quidel
How does the test work?
It’s as easy as Swab, Swirl, Dip, and See Results. Following a gentle nasal swab sample, the QuickVue® At-Home OTC COVID-19 Test strip is mixed into the reagent solution where it rests for 10 minutes. After 10 minutes, the strip is removed, and individuals can review the results immediately.
How long does it take to get results?
Results are available in the privacy of your own home in as little as 10 minutes.
Is a prescription needed to purchase the test?
You do not need a doctor’s prescription to purchase and perform this test.
When will the test be available for purchase?
Quidel intends to begin distribution of the QuickVue® At-Home OTC COVID-19 Test for over-the-counter sales in the coming weeks. Look for the product in late April.
Where can the test be purchased?
The test will be available over-the-counter soon in the United States. Quidel will announce partnerships in the coming weeks.
How much will the test cost?
The final cost is yet to be determined but will reflect a price that makes frequent testing accessible to as many people as possible. A spokesperson for the company notes the hope is for the product to retail under $30.
Is this test recommended for the asymptomatic consumer?
The QuickVue® At-Home OTC COVID-19 Test is intended for use in individuals with symptoms or other epidemiological reasons to suspect a COVID-19 infection.
Do the tests have an expiration date?
QuickVue® At-Home OTC COVID-19 Test has a 12-month shelf life. The expiration date will be clearly labeled on the test kit packaging.
How do you self-test?
The test uses a gentle self-collected anterior nasal (nares) swab sample to determine a positive or negative COVID-19 result. The swab is swirled in a tube of reagent solution, then removed, before a test strip is inserted. After ten minutes, you can take the strip out of the tube and see your results.
 2. Abbott
2. Abbott
- Abbott BinaxNOW (multiple configurations)
- Abbott BinaxNOW COVID-19 Antigen Self Test – authorized for OTC at-home serial screening
- Abbott BinaxNOW COVID-19 Ag Card 2 Home Test – authorized for OTC at-home serial screening with telehealth proctor
- Abbott BinaxNOW COVID-19 Ag 2 Card – authorized for POC serial screening without a prescription
BinaxNOW™ COVID-19 Ag Self Test for detection of COVID-19 infection. This new indication allows individuals with or without symptoms to have access to this test without a prescription.
Abbott will begin shipping to major food, drug, and mass merchandiser retailers in the coming weeks and expect the test to be available through some of their online store websites.
The test can be used on children as young as two years old when samples are collected by an adult and for all people aged 15 years or older, bringing the country’s most extensively studied and widely used rapid antigen test to nearly everyone in the U.S. The test will come in a two-count box to meet serial0020testing requirements.
Using the test will be simple, even for people who have never tested themselves. People will only need to perform a minimally invasive nasal swab (not the deep nasopharyngeal swab). All materials required to complete the test (swab, test card, and reagent solution) will come in the box.
3. BD Veritor System for Rapid Detection of SARS-CoV-2 – authorized for POC serial screening with a prescription.
These tests had been previously authorized by the agency (some under different names) to test those with COVID-19 symptoms. Now testing of asymptomatic individuals can be done more easily.
This is all good news for those who wish to do at-home tests and for schools, workplaces, communities, and others to establish screening programs.